Why should one use Borates
Adding borates to your pool can improve your experience with your pool, but is completely optional.
The key benefit of adding borates to your pool is to help slow pH increase. If you do plan to use borates, wait until everything else is settled down before adding borates, especially TA and PH, and you have a good understanding of how your pool chemistry works.
The only situations where we specifically recommend using borates are for pools with a negative edge or other very large water feature creating huge amounts of aeration and for spas using the dichlor and then bleach method.
All that said, borates have proven popular with many pool owners.
Benefits of using Borates
- More stable pH due to slowing pH increase
- Helps prevent scaling in a SWG cell
- Silky water feel
- More water sparkle
The Borate Pool Opening in Aqua Magazine discusses how borates can limit pH rise while a pool is closed for the winter. High pH while a pool is closed can cause scaling. Those that find their pools with very high pH at Spring opening may benefit from borates, with the pool open or closed.
Borates buffering pH rise
1÷(1+10^(pKa – pH)) = borate percentage.
1÷(1+10^(9.15 – pH)) X 100 = borate percentage.
pH.....Borate.....Boric Acid.
7.2.....1.1%..........98.9%
7.4.....1.7%..........98.3%
7.6.....2.7%..........97.3%
7.8.....4.3...........95.7%
8.0.....6.6...........93.4%.
As you can see from this chart, most of the borate is in the form of boric acid, which contributes to the total acidity.
Total acidity is the same concept as total alkalinity, but total acidity buffers pH rise, whereas total alkalinity buffers pH drop.
Borates at 50 ppm provide as much protection from pH rise as 221 ppm of TA provides against pH drop.
Downfalls of Borates
- Additional expense
- Concerns about risks to pets
- Not a magical potion to solve all pool woes
How do Borates Affect a SWG?
Borates can act as a mild algaecide, so people usually see that “boost” in efficiency because borates can make it harder for algae to replicate. Borates is also a strong buffer against high pH changes inside the cell as the pKa is around 9.0 for the boric acid/borate ion buffer system. Keeping the pH in check means that calcium scaling is less likely, and thus, the cell can run without getting coated with scale. Scale reduces the efficiency of the cell.[1]
Hydroxide is produced in the cell.
2H2O --> H2 + 2OH-
2 water –> Hydrogen gas + 2 hydroxide.
The hydroxide converts bicarbonate to carbonate.
HCO3- + OH- --> H2O + CO3^2-
Bicarbonate + hydroxide --> water + carbonate.
Then, the carbonate connects to calcium and you get calcium carbonate.
Ca^2+ + CO3^2- --> CaCO3
Calcium + carbonate --> calcium carbonate.
Boric acid protects from pH rise by accepting and binding to the hydroxides produced in the cell due to the production of hydrogen.
B(OH)3 + OH- --> B(OH)4-
Boric acid + hydroxide --> Borate.
So, it's really boric acid and total acidity that provides the protection from pH rise and cell scaling.
How to add Borates to your Pool
We recommend maintaining borates between 30 and 50 ppm.
Borates leave the water primarily through splash out and backwashing. [2] Borates are also lost when draining the pool during winter closings. Borates do not degrade.
That normally means checking and raising the borate level to around 50 each spring at the start of swimming season, so that it will still be above 30 come fall.
Before you start on borates, adjust your TA level toward the low end of the appropriate range for your pool type. See the Recommended Levels chart for appropriate levels.
There are two approaches to adding borates to the pool: boric acid, or a combination of Borax and muriatic acid. Using boric acid is just slightly more expensive, in most cases, and much easier. Borax and muriatic acid takes more effort and handling that much acid is just slightly risky, but saves just a little money if you shop carefully.
How to Add Borates Using Boric Acid
Calculate, using PoolMath, how much Boric acid you will need for 50 ppm.
Boric acid can be purchased from DudaDiesel and The Chemistry Store. Granular is much easier to work with than powdered. Technical grade is fine.
Distribute the boric acid across the surface of the pool. Keep the pump running for at least one hour after adding boric acid, and then test the pH and adjust if needed.
Boric acid will lower the pH slightly. Usually the pH change is small enough that no further adjustment is required.
Proteam Supreme Plus is Sodium Tetraborate Pentahydrate and will significantly raise your TA while adding borates to the water. We recommend you use Boric Acid, which adds a minimal amount to your total alkalinity.[3]
Do Not Use Roach and Ant Killer for Boric Acid
Roach and Ant killer can contain boric acid and labels may say it is 99% boric acid and 1% inert. However, they will be very fine powders meant for dusting entryways and such. These types of insecticides will also contain anti-caking agents so that they are free flowing as well as many containing diatomaceous earth (DE) to act as an abrasive which removes the waxy coating from the insect's exoskeleton. Without the waxy coating, the insect dessicates from water loss.[4]
DE (uncalcined) and some anti-caking agents are not considered hazardous (in fact, uncalcined DE is a food additive), and therefore are not reportable in the MSDS. That is why they can say it's 100% boric acid powder even though it might be 60% boric acid and 40% other inert ingredients.
Also the insecticide can have a blue dye added to it so you can see where you’ve sprayed it as a bug killer.[5]
How to Add Borates Using Borax and Muriatic Acid
Borax can be found at grocery stores and muriatic acid at hardware stores. Make sure you check the strength of the muriatic acid, using half strength acid when you thought it was full strength 31.45% can lead to problems with pH.
Calculate, using PoolMath, how much Borax and Muriatic Acid you will need for 50 ppm. Verify the amounts using PoolMath after purchase and double check the weights on the Borax boxes and strength of the Muriatic acid. (Is there only one size Borax box?)
The process for adding the Borax and Muriatic Acid is:
- pre-dissolve 3 1/2 boxes (Do we want to specify box or weight?) of Borax in a bucket
- pour one gallon of 31.45% muriatic acid slowly in front of a return jet with the pump running
- pour the dissolved Borax in the bucket slowly into the pool
- brush the entire pool if you see any undissolved Borax to mix it in and get it dissolved.
- For pools smaller than 10,000 gallons it is better to add a half gallon of 31.45% muriatic acid followed by about 1 3/4 boxes of Borax each time.
Repeat the process (at what interval?) until you have added the correct total amounts to the pool. The final dose will, of course, be smaller.
24 hours later, test the pH and adjust as needed.
How to Test for Borates in Pool Water
Borate Test Strips
We usually do not recommend using test strips, but with borates the exact level is not *super* critical and just needs to be in the 30 to 50 ppm range. A simple solution is to use Taylor Borates Test Strips. The test strips are an inexpensive choice for testing borates.
TFP Borates Drop Test
For a more accurate TFP test approach, the drop test is more accurate.
Equipment Needed
- SpeedStir (is really, REALLY useful!!! We would not recommend performing this test without a SpeedStir)
- 50mL Beaker (can be purchased on Amazon HERE)
- A 1/8th teaspoon measure (can be purchased on Amazon HERE)
The 50mL beaker can sit on the SpeedStir allowing easy mixing of reagents.
Chemicals Needed
- Taylor R-0007 - Chlorine neutralizer reagent
- Taylor R-0009 - Sulfuric acid reagent
- Taylor R-0010 - Sodium hydroxide calcium buffer reagent
- Bromothymol Blue (BTB) – From Amazon HERE
- Mannitol Powder - From Amazon HERE
Borate Drop Test Procedure
- Collect a 50mL sample of pool water, and add to the 50mL beaker.
- Confirm you have 50mL of water in the beaker.
- Add 2 drops of R-0007 to neutralize the chlorine.
- Add enough BTB until the water turns to an easily visible blue color. The volume of BTB applied here will not affect the results of the test; it's just an indicator. We recommend using .5mL of BTB to help with identifying the color transitions.
- Add enough R-0009 dropwise to lower pH. You want the indicator dye to transition from blue to blue-green to yellow-green to straw yellow. Straw-yellow is the color one wants to see. Good results can be used by dividing your TA by 10 and add 2. If your TA is 100, then (100/10)+2 = 12 drops. [6]
- Now add up to 2 drops of R-0010 until the water just turns pale blue. This is the hard part - you want to get the indicator dye to just turn blue, like a baby-blue and not go all the way back to a deeper blue. Typically, 1 or 2 drops is all that is needed. Stop at 1 drop if the sample turns blue. NOTE THIS COLOR IN YOUR BRAIN, you will use it in step 8.
- Add 3 level spoonful’s (3/8 teaspoon total) of Mannitol. If the water has boron in it, then the sample will turn yellow again. Let it spin for 30 seconds to allow the mannitol to dissolve.
- Slowly add R-0010 drops until the water transitions from straw yellow to greenish blue to the baby blue color from step 6. Adding the R-0010 slowly gives time for the solution to mix and transition colors. Once the color transitions to the light baby blue from step 6, you are done. Record how many drops of R-0010 are used in this step.
- Calculate ppm boron/borates by multiplying the number of drops of R-0010 used only in step 7 by 4ppm/drop, that is your Borates level in ppm.[7]
What the Borate Drop Test Should Look Like
Adding .5mL of BTB:
After adding BTB:
After adding R-0009:
After adding R-0010:
After adding Mannitol:
After adding R-0010 the second time, what the end point should look like:
Notes on Borate Drop Testing
- Dechlorinating the sample is important. The bromothymol blue (BTB) dye is sensitive to chlorine and will be bleached by it. 2 drops of R-0007 are usually more than enough for FC in normal ranges.
- The R-0010 reagent (sodium hydroxide) has a very STRONG effect on raising the pH. So, it should not take much to go from pale yellow to blue in Step 5. You don't want to overshoot this and add lots of R-0010 in this step. It would be easier if the R-0010 were less concentrated but we're stuck with what we have available.
- Yes, you have to try to remember the shades of blue unfortunately. This is a titration test where you are trying to measure something called the "equivalence point", i.e., the exact pH value where there's a sharp transition from yellow to blue. This is very easily seen with a pH probe (which is what you would use in a lab) but it's much harder to do with a visual color determination. What you're really after is that exact point when the BTB changes from a greenish-blue color to baby-blue as that is the point in the pH curve where the sharpest change occurs. If anyone is interested in the chemistry details, this website has a decent explanation - Titration Fundamentals - Chemistry LibreTexts
Borates and Health Concerns
Borates have in the past had a reputation for being unsafe for both humans and pets, mostly dogs. While very high levels can pose some risks TFP Suggested Levels are safe for both. A 100 pound dog would need to drink 8 liters (over 8 quarts) of 50 ppm borate pool water every day just to be at the No Observed Adverse Effect Limit (NOAEL). The level seen for first symptoms is 3 times higher than this amount. And this is literally drinking every day since borates are excreted from the body and do not accumulate so the daily intake level where problems would occur is that which is higher than the rate at which the body flushes out borates.
Boron is an essential nutrient so the body takes in what it needs and excretes the rest, though this process has its limits which is why almost anything is toxic if given in high enough doses. This excretion process is fairly efficient for mammals that use urine primarily to excrete excess nitrogen as urea, but for insects they excrete solid uric acid so do not excrete boron efficiently which is why boron is far more toxic to them.[8]
Some threads on Borate safety:
Are Borates an Algaecide?
While Borates tend to have a minimal effect as an algaecide one shouldn’t utilize them to prevent algae. The boron concentration that that is required to noticeably reduce the risk of algae[9] is far higher than what is generally considered safe so we generally don’t utilize them to prevent algae, we only focus on the benefits for buffering pH changes.
What are the algaestatic properties of borates
The algaestatic properties of borates are over-stated at 50 ppm. Borates act more as an "inhibitor" than a biocidal agent. In other words, boron in the water at 50 ppm interferes with certain cellular processes inside the algae (likely inhibiting key enzymes that contain diol organic structures). By causing this interference, the algae is either slowed in its replication or its reproduction is stopped.[10]
Borates only become truly algaestatic at concentrations above 100 ppm (and closer to 200 ppm), making the water unhealthy for large mammals and humans to swim. Above 100 ppm, there could be chronic toxic effects on very small children (babies) and pets (dogs or cats) that might accidentally (or intentionally) ingest pool water.
Is Borax and Baking Soda the same thing
Borax and Baking Soda are two different chemicals. Borax will increase the pH of the water without increasing the total alkalinity. Baking soda, on the other hand, will help increase your total alkalinity.
Borates and Adjusted Alkalinity
Adjusted TA = TA – (CYA X CYA C.F) – (Borate x Borate CF)
Borate C.F (correction factor) based on pH.
pH.......CF
7.2.....0.051
7.4.......0.0786
7.6......0.1248
7.8......0.1989
Cyanuric Acid correction factor based on pH.
pH........CF
7.0.......0.22
7.1.......0.24
7.2.......0.26
7.3.......0.28
7.4.......0.30
7.5.......0.32
7.6.......0.33
7.7.......0.34
7.8.......0.35
7.9.......0.36
For example, if the pH = 7.6, TA = 90, Borate = 50 and CYA = 70, the adjusted alkalinity is 90 - (70 x 0.33) – (50 x 0.1248) = 60.66.
A Pool Care Experts View of Borates in Pools
The following was written by Kim Skinner, aka OnBalance, and published with his permission.
A Bit of History About Borate Use in Pools
There are a lot of bold claims about the benefits of adding borate (boron) to swimming pools. Are they true? Well, some are true, and some are not.
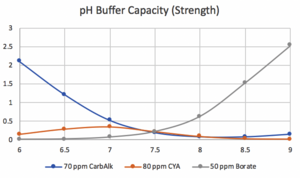
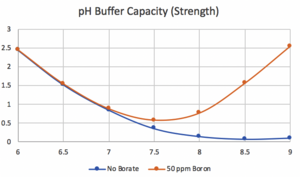
The following information about borate in pools comes from Kim Skinner and John Cardall, who added borax (borate) to pools as early as 1966 as teenagers while working for Pool Chlor Inc., a pool service company in southern California. At that time, Pool Chlor maintained more than 1000 residential pools and used chlorine gas as their primary sanitizer. As many know, chlorine gas is very acidic and lowers the pH when added. In 1960, two chemists who owned Pool Chlor realized that (carbonate) alkalinity and CYA were suitable buffers at pH below 7.2 but not highly effective at pH 8.0. They searched for a chemical that would increase buffering at a higher pH (8.0-8.2) and learned that adding borax would help. The graphs below illustrate the buffering differences.
It was soon determined that about 50 ppm of boron was helpful, along with an adequate amount of total alkalinity, to buffer the generated acid properly. With a starting pH of about 8.0 to 8.2, the presence of borate improved buffering (neutralizing) the acid produced by the chlorine gas.
Thus, Pool Chlor began adding borax systematically to their pools in about 1962. (That is more than 20 years before a chemical company in Florida applied for a patent).
Additional Benefits of Borate
Borate also helps buffer against a rising pH when alkaline products such as soda ash, bleach, or cal hypo are added. One benefit is that, under certain conditions, such as hard water, the water is not as likely to turn cloudy when those products are added to water.
Interestingly, some eye-soothing solution products contain boric acid and sodium borate and have a pH of about 8.0. Customers reported that the pool water containing borate results in less eye irritation. Some pool owners claimed the water was so clear that it “sparkled.” Truthfully, we think pool water can sparkle without borate, but it may also play a role in that! (A possible reduction in surface tension may be why). There are also reports (and some supporting science) that having borate in water helps to minimize calcium build-up in SWCG units.
What Claims are Not True?
Borate will NOT reduce chlorine costs by 30% to 50% as claimed. It is generally known that residential pools lose, on average, about 1 to 2 ppm of chlorine per day. Several service companies say residential pools typically lose the same amount of chlorine daily, whether they contain borate or not. And the suggestion to maintain lower chlorine levels when using borate is risky and problematic.
It is incorrect to suggest that Borate “prevents” or “stops” the pH from rising. While borate can somewhat slow down or reduce the pH rise when alkaline chemicals are added, it is only temporary. Generally, the pH will eventually rise above 7.6 (and up to 8.3) if the (carbonate) alkalinity is 80 to 120 ppm and if no acidic chemicals are being added. That is the general reality of water chemistry.
It is misleading to suggest that borates (50 ppm boron) will eliminate or prevent algae. Pool water requires an adequate amount of sanitizer to kill and prevent algae. Borate will not do that. When sanitizers are no longer present, the water will soon grow algae and bacteria. However, borate is an algae-stat (not an algaecide) and can inhibit and slow down algae growth, reducing chlorine costs.
Borate does not eliminate or prevent calcium nodules. Calcium nodules are caused by excessive shrinkage cracks and/or delamination (bonding failure), not by out-of-balance pool water. And borate does nothing to change that. Borate has some good benefits (by adding borax or boric acid). But we should not tarnish its’ appeal and benefits by making false claims, exaggerations, or misleading disinformation.
We thank Richard Falk for his input and the two graphs.
- ↑ https://www.troublefreepool.com/threads/borates-and-swg.194373/post-1713562
- ↑ https://www.troublefreepool.com/threads/do-borates-degrade.261349/post-2285195
- ↑ https://www.troublefreepool.com/threads/proteam-supreme-plus-raised-ta.214268/post-1875423
- ↑ https://www.troublefreepool.com/threads/boric-acid-the-easy-way.37456/post-832022
- ↑ https://www.troublefreepool.com/threads/boric-acid-no-no.196020/post-1728248
- ↑ https://www.troublefreepool.com/threads/new-borate-drop-test-at-piscines-apollo-vs-test-strip.33440/post-1304775
- ↑ https://www.troublefreepool.com/threads/new-borate-drop-test-at-piscines-apollo-vs-test-strip.33440/post-1270754
- ↑ https://www.troublefreepool.com/threads/are-borates-safe-to-use.14157/post-464493
- ↑ https://www.troublefreepool.com/threads/other-things-that-claim-to-reduce-chlorine-usage.65703/#post-557324
- ↑ https://www.troublefreepool.com/threads/borax-as-algestat.128544/post-1136923