Recently the color of my pH samples has gotten more intense and it makes it difficult to accurately determine what the proper color match is on my Taylor test kit. Nothing has changed in the chemicals I'm using however the water temp has gone from 74 deg. to 64 deg. in the last 12 days. I've found that if I only use four drops of solution I get a much more accurate color reading than if I use 5 drops. Is this still accurate? Can water temperature affect the color this way?
Below are a couple of pictures demonstrating the difference.
Thanks,
Gary
5 drops
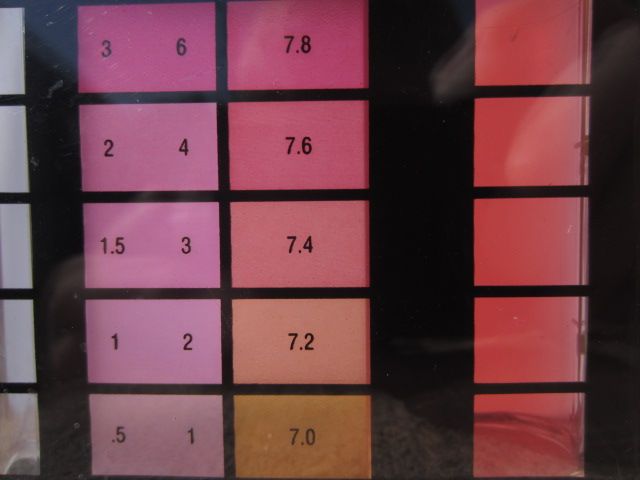
4 drops
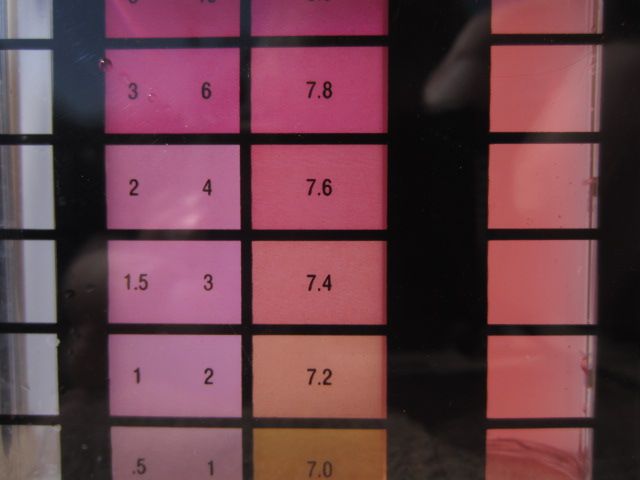
Below are a couple of pictures demonstrating the difference.
Thanks,
Gary
5 drops

4 drops


