- Sep 7, 2022
- 262
- Pool Size
- 16500
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
I just had my Pool&Spa resurfaced with StoneScapes Mini Pebble. The start up procedure calls for reducing Alkalinity to 80ppm and reducing pH to between 7.2 and 7.6 on day One after Pool is filled.
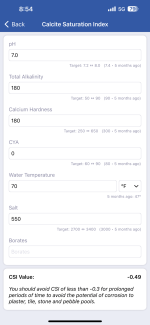
The issue is my potable fill water has a pH which is in the range, but the Alkalinity is 180ppm.
Fill Water Results:
pH: 7.5 - 7.6
Total Alkalinity: 180ppm
Calcium Hardness: 180ppm
Salt: 410ppm (digital meter)
After the fill, I added 2 gallons of 14.5% Muriatic acid which decreased the pH to below 7.0 and decreased Alkalinity to 130ppm
My question is, when starting up new plaster, is it okay to reduce Alkalinity in the usual slow way, whereby you add enough acid to decrease alkalinity while only decreasing pH to 7.2, then aerate to bring back pH while retaining the Alkalinity levels, and repeat this process until Alkalinity is in range?
The instructions make it seems like you are supposed to do this in one day, on day 1 of start up. Won’t adding that much acid in one go to decrease Alkalinity from 180ppm to 80ppm decrease pH to severe levels?
The issue is my potable fill water has a pH which is in the range, but the Alkalinity is 180ppm.
Fill Water Results:
pH: 7.5 - 7.6
Total Alkalinity: 180ppm
Calcium Hardness: 180ppm
Salt: 410ppm (digital meter)
After the fill, I added 2 gallons of 14.5% Muriatic acid which decreased the pH to below 7.0 and decreased Alkalinity to 130ppm
My question is, when starting up new plaster, is it okay to reduce Alkalinity in the usual slow way, whereby you add enough acid to decrease alkalinity while only decreasing pH to 7.2, then aerate to bring back pH while retaining the Alkalinity levels, and repeat this process until Alkalinity is in range?
The instructions make it seems like you are supposed to do this in one day, on day 1 of start up. Won’t adding that much acid in one go to decrease Alkalinity from 180ppm to 80ppm decrease pH to severe levels?